Protons neutrons and electrons practice – Embarking on the study of protons, neutrons, and electrons practice, this comprehensive guide unravels the fundamental building blocks of matter, delving into their properties, interactions, and practical applications.
This exploration illuminates the distinct characteristics of protons, neutrons, and electrons, examining their location within the atom and the impact of their mass, charge, and location on their behavior.
1. Subatomic Particles
Protons, Neutrons, and Electrons
Subatomic particles, the fundamental building blocks of matter, include protons, neutrons, and electrons. Each particle possesses unique characteristics that determine its behavior and interactions within the atom.
1.1. Fundamental Characteristics
- Protons:Positively charged particles located in the nucleus of an atom, with a mass of approximately 1 atomic mass unit (amu) and a charge of +1e.
- Neutrons:Neutral particles also located in the nucleus, with a mass of approximately 1 amu but no charge.
- Electrons:Negatively charged particles that orbit the nucleus, with a mass of approximately 1/1836 amu and a charge of -1e.
1.2. Location Within the Atom
Protons and neutrons reside in the nucleus, which is the central core of an atom. Electrons, on the other hand, occupy energy levels or orbitals that surround the nucleus.
2. Properties of Subatomic Particles
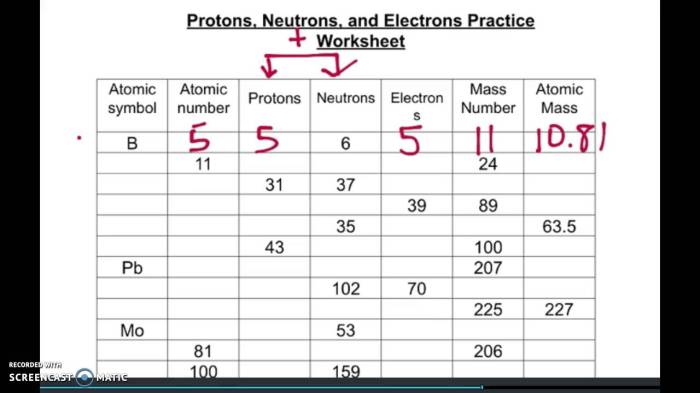
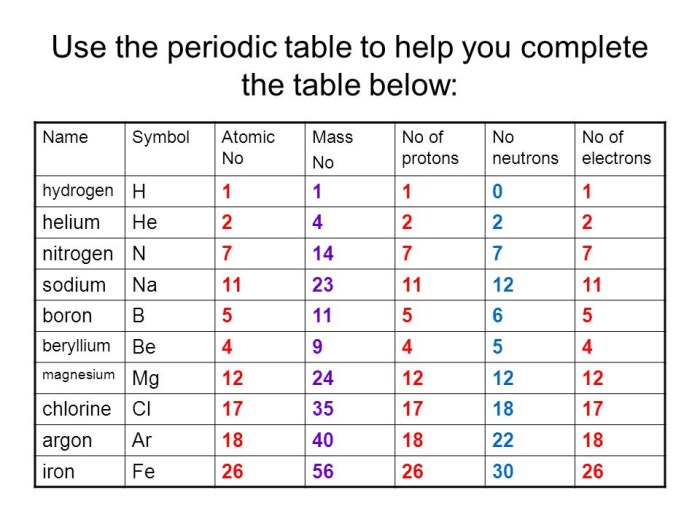
2.1. Comparison of Mass, Charge, and Location, Protons neutrons and electrons practice
| Property | Proton | Neutron | Electron |
|---|---|---|---|
| Mass (amu) | ~1 | ~1 | ~1/1836 |
| Charge (e) | +1 | 0 | -1 |
| Location | Nucleus | Nucleus | Orbits nucleus |
2.2. Impact on Behavior
The mass, charge, and location of subatomic particles significantly influence their behavior:
- Mass:The mass of a particle affects its inertia and gravitational interactions.
- Charge:The charge of a particle determines its electromagnetic interactions with other charged particles.
- Location:The location of a particle within the atom influences its potential energy and participation in chemical reactions.
3. Interactions Between Subatomic Particles
3.1. Electromagnetic Force
The electromagnetic force is a long-range force that acts between charged particles. Protons and electrons have opposite charges, resulting in an attractive force that binds them together in atoms. Neutrons have no charge, so they do not participate directly in electromagnetic interactions.
3.2. Strong Nuclear Force
The strong nuclear force is a short-range force that acts between protons and neutrons within the nucleus. It is much stronger than the electromagnetic force and overcomes the repulsive electrostatic force between protons.
3.3. Contribution to Stability and Structure
These forces play a crucial role in maintaining the stability and structure of atoms. The electromagnetic force holds electrons in orbit around the nucleus, while the strong nuclear force binds protons and neutrons together in the nucleus.
4. Practical Applications of Subatomic Particles: Protons Neutrons And Electrons Practice
4.1. Nuclear Energy
Protons and neutrons are used in nuclear reactions, which release enormous amounts of energy through processes like nuclear fission and fusion. This energy is harnessed in nuclear power plants to generate electricity.
4.2. Medical Imaging
Electrons and radioactive isotopes are utilized in medical imaging techniques such as X-rays and PET scans. These techniques allow medical professionals to diagnose and treat diseases by visualizing the internal structures and functions of the body.
4.3. Material Science
Subatomic particles are essential in understanding and manipulating the properties of materials. By studying the interactions between protons, neutrons, and electrons, scientists can design materials with desired properties for various applications, such as electronics, batteries, and catalysts.
Essential FAQs
What is the fundamental difference between protons and neutrons?
Protons possess a positive charge and reside in the atom’s nucleus, while neutrons carry no charge and are also found in the nucleus.
How do electrons contribute to the chemical properties of elements?
Electrons, located in orbitals surrounding the nucleus, determine an element’s chemical reactivity by participating in the formation and breaking of chemical bonds.