Maxwell boltzmann distribution pogil answers – Embark on a scientific odyssey with our comprehensive guide to Maxwell-Boltzmann distribution POGIL answers, where we unravel the secrets of molecular motion and its profound implications across diverse fields. Join us as we delve into the fascinating world of statistical physics, exploring the intricate relationship between particle energy and distribution, and empowering you with the knowledge to tackle POGIL activities with confidence.
Maxwell-Boltzmann Distribution
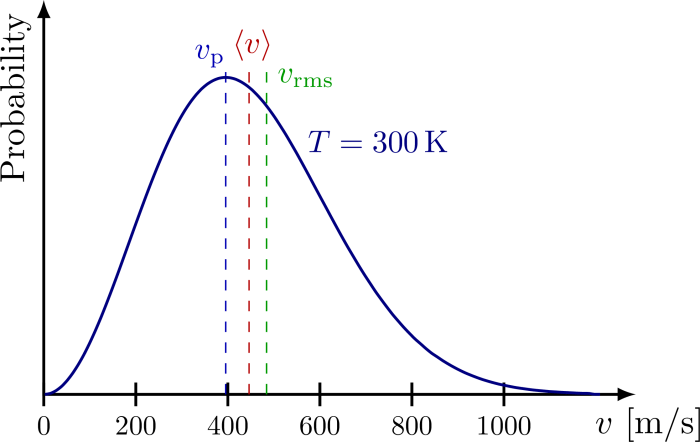
The Maxwell-Boltzmann distribution is a statistical distribution that describes the distribution of molecular velocities in a gas at a given temperature. It is based on the assumption that the gas molecules are in constant random motion and that their velocities are distributed according to a Gaussian distribution.
Relationship to Kinetic Energy
The Maxwell-Boltzmann distribution is directly related to the kinetic energy of the gas molecules. The mean kinetic energy of a gas molecule is proportional to the temperature of the gas, and the distribution of velocities around this mean is described by the Maxwell-Boltzmann distribution.
Example
In a real-world scenario, the Maxwell-Boltzmann distribution can be used to predict the distribution of velocities of molecules in a gas at a given temperature. This information can be used to design experiments, such as those involving the measurement of gas viscosity or thermal conductivity.
Maxwell-Boltzmann Distribution in POGIL
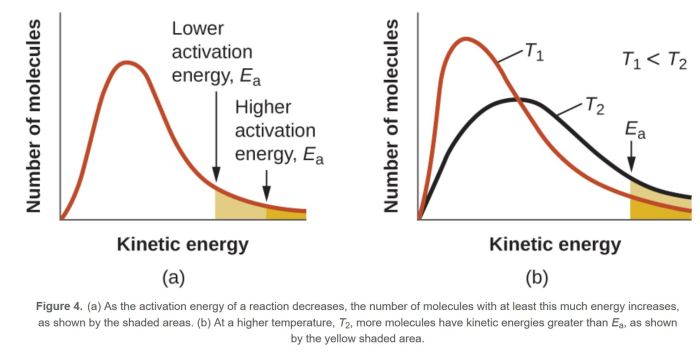
The Maxwell-Boltzmann distribution is a valuable tool in POGIL activities because it allows students to analyze data and make predictions about the behavior of gases.
Steps for Using the Distribution
- Collect data on the velocities of gas molecules.
- Plot the data on a histogram.
- Fit the histogram to a Maxwell-Boltzmann distribution.
- Use the distribution to make predictions about the behavior of the gas.
Example Activity
One POGIL activity that utilizes the Maxwell-Boltzmann distribution is the “Gas Viscosity” activity. In this activity, students collect data on the viscosity of a gas at different temperatures. They then use the Maxwell-Boltzmann distribution to predict the viscosity of the gas at a new temperature.
Applications of the Maxwell-Boltzmann Distribution: Maxwell Boltzmann Distribution Pogil Answers
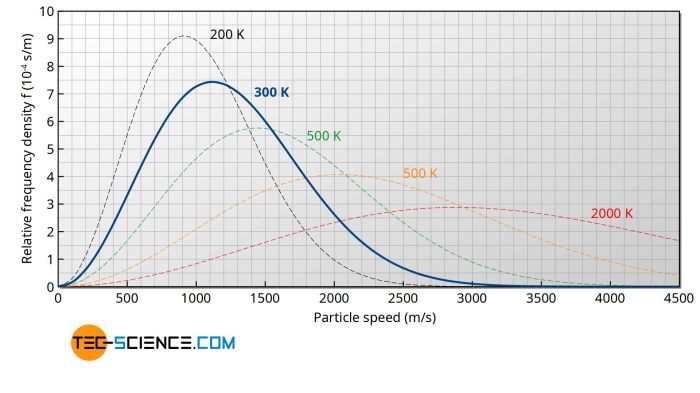
The Maxwell-Boltzmann distribution has a wide range of applications in various fields.
Applications
- Physics:Calculating the transport properties of gases, such as viscosity and thermal conductivity.
- Chemistry:Predicting the rates of chemical reactions and the equilibrium concentrations of reactants and products.
- Engineering:Designing engines, turbines, and other devices that involve the flow of gases.
Examples
For example, the Maxwell-Boltzmann distribution can be used to design more efficient engines by optimizing the flow of gases within the engine.
Limitations
However, the Maxwell-Boltzmann distribution has limitations and may not be applicable in all situations. For instance, it does not account for quantum effects or the interactions between molecules at high densities.
Advanced Concepts Related to the Maxwell-Boltzmann Distribution
Boltzmann Factor, Maxwell boltzmann distribution pogil answers
The Boltzmann factor is a mathematical expression that describes the probability of a particle occupying a particular energy state. It is directly related to the Maxwell-Boltzmann distribution.
Thermodynamic Properties
The Maxwell-Boltzmann distribution can be used to calculate thermodynamic properties, such as entropy and free energy, of a system of particles.
Statistical Mechanics
The Maxwell-Boltzmann distribution is closely connected to statistical mechanics, which provides a framework for understanding the behavior of large systems of particles.
Essential FAQs
What is the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution is a statistical model that describes the distribution of molecular velocities in a gas. It predicts the probability of finding a molecule with a given velocity at a given temperature.
How is the Maxwell-Boltzmann distribution used in POGIL activities?
POGIL activities often involve analyzing data related to molecular motion. The Maxwell-Boltzmann distribution can be used to model this data and extract information about the temperature and energy distribution of the molecules.
What are the applications of the Maxwell-Boltzmann distribution?
The Maxwell-Boltzmann distribution has applications in a wide range of fields, including physics, chemistry, and engineering. It is used to study phenomena such as diffusion, thermal conductivity, and chemical reactions.